 Waukeshahealthinsurance.com-
Waukeshahealthinsurance.com-
–
The US Centers for Disease Control and Prevention issued an advisory on Tuesday about the dangers of fake or incorrect Botox injections Dangerous fake versions of the product have been found In many states.
The CDC, the U.S. Food and Drug Administration, and state and local officials are working to investigate a cluster of at least 22 women who reported adverse reactions to fake Botox injections, injections from unlicensed or untrained providers, or shots in unsanitary settings. Care settings such as spas or private homes Official CDC health advice.
As of Thursday, those incidents had been reported in California, Colorado, Florida, Illinois, Kentucky, Nebraska, New Jersey, New York City, Tennessee, Texas and Washington. Of those who reported symptoms, 11 were hospitalized, the CDC said, but none died.
Patients ages 25 to 59 reported their symptoms between November and March, and most — 91% — received Botox for cosmetic purposes, according to the CDC. Symptoms include blurred vision, droopy eyelids, dry mouth, slurred speech, shortness of breath, fatigue and weakness. Seven of these tested positive for botulism. Test results were negative for six, one is still pending.
These incidents appear to be related to products purchased from unlicensed sources and then administered by unlicensed or licensed suppliers. Previous FDA announcement. The agency warned health professionals that buying and administering counterfeit products could put patients' health at risk.
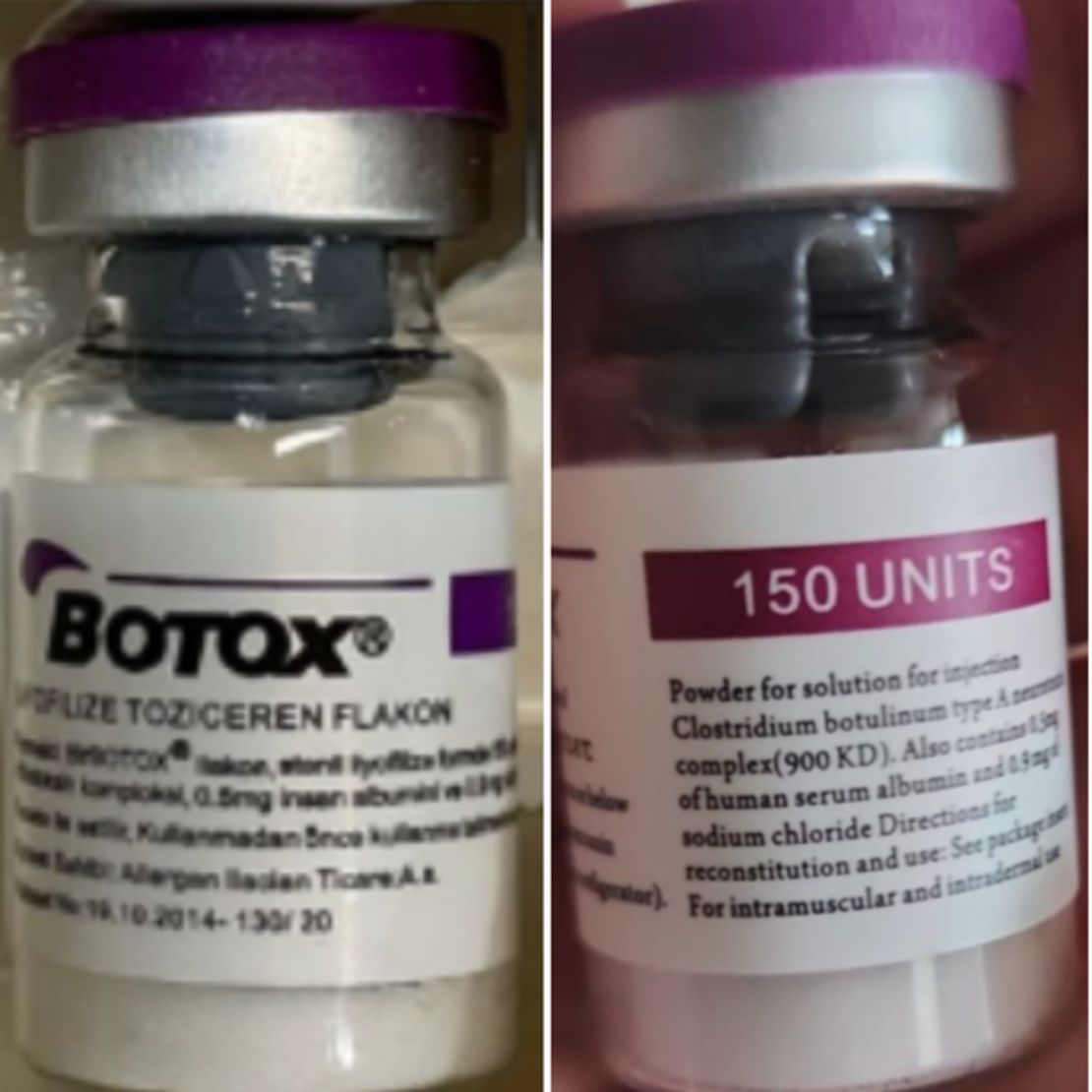
Counterfeit Botox products can be identified by the lot number C3709C3 on the outer carton and vial, according to the FDA.
The outer carton may list the active ingredient as “Botulinum Toxin Type A” instead of “OnabotulinumtoxinA”. The carton and vale may refer to 150-unit doses, which is not a dose made by the companies AbbVie or Allergan, the FDA said, and the outer carton may contain non-English language.
In an advisory Tuesday, the CDC noted that botulinum toxin, also known as Botox, can cause adverse effects when it spreads to the injection site.
“Botulism is a disease caused by botulinum toxin that circulates in the blood and affects the injection site. There may be signs of local adverse effects of botulinum toxin injections, especially in the head and neck, and between the first symptoms of botulism.
“Clinics and health departments should consider the negative effects of botulinum toxin injections in patients who present with local paralysis,” the recommendation says. “If botulism is suspected, clinicians should contact their state, tribal, local or territorial health department immediately.”
According to the CDC, botulism can be treated with antitoxin. That is why it is important to get medical help at the beginning of the disease and quickly. If the disease is not treated, it can lead to paralysis and other problems.
“Botulinum toxin should be administered only by licensed providers, using only FDA-approved doses of botulinum toxin, preferably in a licensed or accredited health care setting,” the CDC advises. “Providers should be trained in the proper administration of botulinum toxin in accordance with state and local requirements.”
In response to reports of fake Botox, the companies behind the FDA-approved versions, AbbVie and Allergan Aesthetics, said in a statement that they are the only authorized suppliers of Botox in the United States.
“Allergan Aesthetics, an AbbVie company, has a comprehensive supply chain safety program to ensure that all products manufactured are safe, secure and sold through authorized distribution channels. All reports of counterfeit products are thoroughly investigated by our team and in cooperation with law enforcement and public health officials when appropriate, the statement said.
Suppliers and consumers can identify authentic Botox products by a physical seal on the packaging, the brand name Botox with the word “onabotulinumtoxinA” and the word “Allergan” on the vial label with a hologram.
Botox is the brand name of botulinum toxin and is used in small doses to treat medical or cosmetic concerns such as severe underarm sweating, chronic migraines, overactive bladder and facial wrinkles.
“Botox and its equivalent brands provide a mild, precise and consistent dose of botulinum toxin, but unregulated products are not manufactured as safely or subject to the same quality assurance, which can lead to overdose or ingestion,” said Dr. Michael Cameron. , President and Founder Cameron Dermatology and assistant professor of dermatology at Mount Sinai Health System in New York; He said last week.
A large amount of botulinum toxin in the body can cause a rare but serious illness botulism or symptoms similar to the disease.
“Symptoms of botulism include muscle weakness, vision changes, slurred speech, trouble moving your eyes. If you start having trouble breathing, it's an emergency,” Cameron said.
“If you're getting Botox for sweat wrinkles, I personally recommend seeing a board-certified dermatologist. If you are taking Botox for your migraines, see a neurologist. If you're taking Botox for your bladder issues, go to your urologist,” he said. “If you feel there is a risk or the pricing doesn't make sense, you can ask to see the bottle and inspect the botox bottle yourself.”
